59 00 f and 101 325 kpa.
Room temperature and pressure values.
The density of air or atmospheric density denoted ρ greek.
The same amount of water vapor results in higher relative humidity in cool air than warm air.
To convert densities to moles per liter multiply by 22 678 cm 3 mol l g.
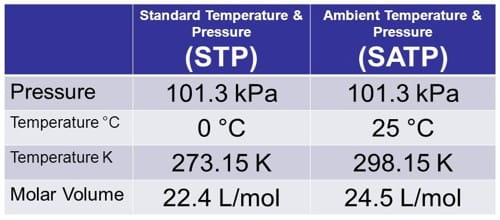
Standard temperature is defined as zero degrees celsius 0 0 c which translates to 32 degrees fahrenheit 32 0 f or 273 15 degrees kelvin 273.
Heat content data heat of vaporization and entropy values are relative to the liquid state at 0 c temperature and 3483 kpa pressure.
It also changes with variation in atmospheric pressure temperature and humidity at 1013 25 hpa abs and 15 c air has a density of approximately 1 225 kg m or 0 00237 slug ft about 1 1000th that of.
Standard temperature and pressure abbreviated stp refers to nominal conditions in the atmosphere at sea level.
Relative humidity rh is the ratio of the partial pressure of water vapor to the equilibrium vapor pressure of water at a given temperature.
This value is important to physicists chemists engineers and pilots and navigators.
Satp standard ambient temperature and pressure is a reference with temperature of 25 o c 298 15 k and pressure of 101 325 kpa.
Colloquially room temperature is the range of air temperatures that most people prefer for indoor settings which feel comfortable when wearing typical indoor clothing.
Nist uses a temperature of 20 c 293 15 k 68 f and an absolute pressure of 1 atm 14 696 psi 101 325 kpa.
Rho is the mass per unit volume of earth s atmosphere air density like air pressure decreases with increasing altitude.
If the same experiment is conducted by him her in austin where mean room temperature and pressure is 21 and 1 014 bar the result of the experiments might significantly vary.
Mean room temperature and pressure in london is 12 and 1 015 bar.
Satp standard ambient temperature and pressure is also used in chemistry as a reference.
1 mole of any gas occupies 22 4 dm 3 at stp standard temperature and pressure taken as 0 c and 1 atmosphere pressure.
At these conditions the volume of 1 mol of a gas is 24 4651 liters.
This is because many scientific experiments particularly of chemistry are influenced by.
Human comfort can extend beyond this range depending on humidity air circulation and other factors.
You may also have used a value of 24 0 dm 3 at room temperature and pressure taken as about 20 c and 1 atmosphere.
In certain fields like science and engineering and within a particular context room temperature can mean different agreed.
To convert heat values to joules per mole values multiply by 44 095 g mol.
The international standard metric conditions for natural gas and similar fluids are 288 15 k 15 00 c.
Relative humidity depends on temperature and the pressure of the system of interest.